Describe the Effector Cells Involved in Humoral Immunity Review Sheet 35
Immunology seems to be 1 of those things that people either love or hate; I think it's fascinating, just I know there volition be a lot of people out there who arroyo the subject field with a mixture of terror, frustration and loathing. To brand the normal immune response less of a horrendous nightmare to learn, I made a summary diagram showing friendly, loveable cartoon immune cells doing what they practice all-time – pwning pathogens. I know it looks pretty complicated, simply don't panic! I'm also going to explain everything step past footstep, then past the stop of this article information technology will all make sense and you'll feel super clever.
Y'all can click on the diagram to overstate it, and delight feel free to download and print it too.
P.Due south. This article is a fleck of a whopper, so while things are press I would nip and get a cup of tea/coffee/your unhealthy revision fuel of choice to ensure you're sufficiently energised before you lot get-go!
Y'all might also exist interested in our medical flashcard drove which contains over 1000 flashcards that cover key medical topics.
Beefcake of the immune organisation
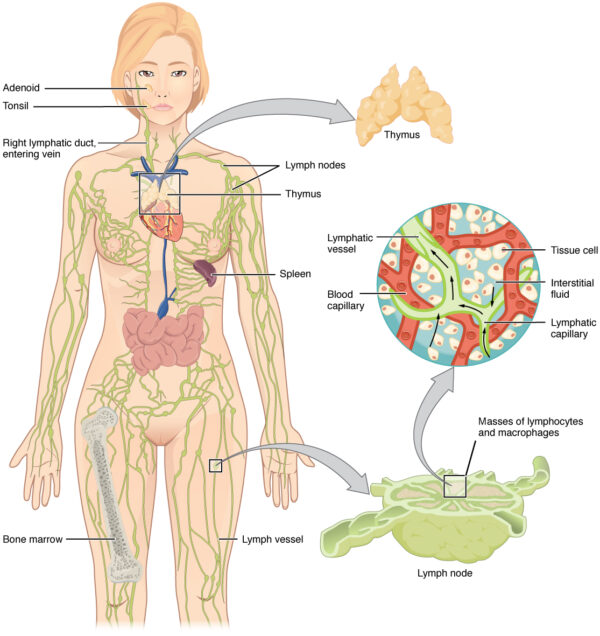
The allowed system is a mobile, circulating system. However, at that place are some fixed anatomical structures that are important to its function:
- allowed cells are made within the os marrow during haematopoiesis
- the thymus gland is situated merely in front of the heart in the mediastinum. It is agile throughout life, but is at its largest in babyhood and decreases in size after puberty. Information technology is where lymphocytes mature and receive their immunological "education" before existence released into the bloodstream.
- mature lymphocytes migrate to lymph nodes, which are pocket-size bean-similar structures situated along the lymphatic vasculature throughout the body. These filter lymph and provide a site for antigen presentation to the adaptive immune organisation. Lymph is then returned to the systemic apportionment via the thoracic duct, which joins with the left subclavian vein.
- the spleen is basically a massive lymph node and is, therefore, another site of antigen presentation to mature lymphocytes. It is part of the reticulo-endothelial system which filters blood and removes onetime cells, tissue debris, pathogens and immune complexes. It likewise stores red blood cells and young monocytes.
- finally, the liver is also a site of antigen presentation and contains its own cohort of phagocytes and lymphocytes. This is a vital part, every bit the liver filters big volumes of potentially contaminated venous blood from the gastrointestinal (GI) tract. It as well synthesises acute phase proteins such as CRP in response to infection.
Barrier mechanisms of the immune arrangement
There are numerous potential ways for pathogens to enter the torso. Humans have therefore evolved several concrete and chemical bulwark mechanisms to prevent the invasion of infective organisms:
- intrinsic epithelial barriers exist between the torso and the outside world. Epithelial cell walls have very tight junctions betwixt them and are therefore hard to penetrate. Examples include the linings of the oral cavity, nasal passages, upper airways, lungs and GI tract.
- the continuous longitudinal flow of air or fluid through most body systems helps to create a flushing action which prevents situations in which leaner could adhere to structures, proliferate and invade
- the moveof mucus by cilia in the lungs also helps foreclose the stagnation of secretions and the adherence of inhaled droplets and particles. Mucus is moved up towards the pharynx, where it is and then swallowed or coughed up.
- desquamation of peel and epithelial cells also prevents adherence of microorganisms
- natural acids persist in many parts of the body, for example, fatty acids on the pare, lysozymes in saliva and hydrochloric acid in the tum
- there are also many natural antibacterial peptides on the skin and the surface linings of the lungs and gut. These include cathelicidins, defensins, proteinase inhibitors and chemokines.
- normal bacterial flora colonising various parts of the torso compete with infective microorganisms, and some also produce antimicrobial substances. For example, vaginal lactobacilli produce lactate, which creates an acidic environment and destroys many potentially infectious organisms.
Cells of the immune organisation
There are many different groups of cells involved in the immune response. Depending on which medical schoolhouse y'all're at, you may exist expected to be able to recognise them on microscopy, then I've included some pictures of actual real cells alongside my giddy cartoon ones.
GRANULOCYTES
A family of white blood cells containing granules in their cytoplasm
NEUTROPHILS
- usually make upwards40-75% of all white claret cells (ii-7.5 x 109/L on a full blood count)
- the first line of defense force against all infections
- deed by phagocytosing invading organisms and presenting antigens to the immune organization
- they have segmented nuclei and their cytoplasm is full of pinky-purple intracellular granules
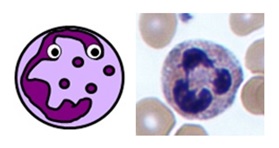
Neutrophil
EOSINOPHILS
- normally make upwardly 1-half-dozen% of white blood cells (0.04-0.44 x ten9/50 on a full blood count)
- they specifically act against multicellular parasites (due east.g. worms) by dissolving their cell surfaces
- they are as well involved in IgE-mediated allergic disorders such every bit asthma
- they have bilobed nuclei andintracellular granules which stain brick reddish with eosin
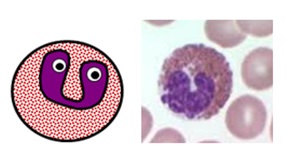
Eosinophil
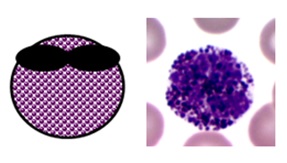
BASOPHILS
- normally brand upwardly 0-1% of white blood cells (≤0.01 ten ten9/50 on a full claret count)
- they are the circulating counterparts of tissue mast cells and are rather mysterious
- they probably have roles in inflammation,parasitic infections and allergic reactions
- mast cells/basophils take an of import role in type ane hypersensitivity reactions through their binding with IgE antibodies
- they take bilobed nuclei and big darkly staining intracellular granules
MONOCYTES & MACROPHAGES
Big cells involved in phagocytosis and antigen presentation
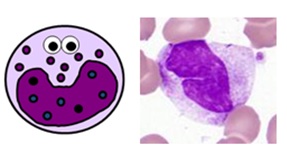
Blood MONOCYTES
- unremarkably make up 2-10% of white blood cells (0.2-0.8 ten ten9/Fifty on a full blood count)
- they are produced in the bone marrow and travel in the bloodstream to their target tissues, where they become macrophages
- they have roles in phagocytosis, antigen presentation and cytokine production
- they are big cells with fine "ground-drinking glass" granules and horseshoe-shaped nuclei
Monocyte
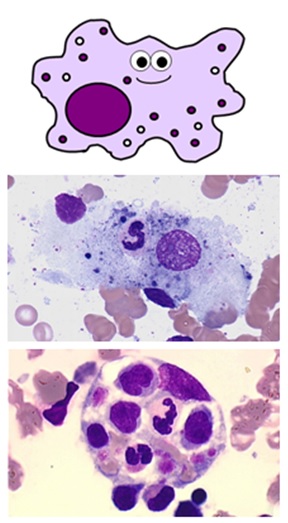
TISSUE MACROPHAGES
- these are tissue cells and therefore aren't found on a total blood count
- they are derived from blood monocytes, which differentiate once they reach their target tissues and express CD14 receptors
- they "tidy up" whatever pathogens, foreign debris and old or dead cells from their tissues usingphagocytosis
- they too perform antigen presentationand tin canactivate retentiveness cells
- like monocytes, they are large cells with horseshoe-shaped nuclei
- the name macrophage means "large eater". As you can see, they can exist quite difficult to identify depending on how much they've eaten!
- they have processes on their cell membranes called pseudopodia which extend around the unlucky item they're about to eat
- in one case internalised, the engulfed material is contained inside a large vesicle called aphagosome
- the phagosome is fused with another vesicle called a lysosome containing eitherreactive oxygen species or enzymes, which suspension downward its contents
- in that location are many types of macrophage which are specifically adapted to different tissues – these include Küpffer cells in the liver, alveolar macrophages in the lungs, osteoclasts in bone and microglial cells in neurones
Macrophages
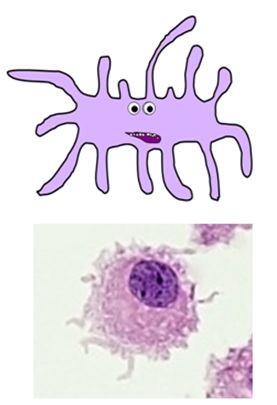
DENDRITIC CELLS
- this complex family of cell types are the main "professional"antigen-presenting cells of the immune system
- they play a vital function in activatingT helper cells and memory cells
- they are formed in the bone marrow and circulate in the bloodstream until they achieve their target tissues, where they are activated by pathogens and differentiate into their mature forms
- they phagocytose pathogens earlier migrating to lymph nodes, where they present antigens on their cell surfaces with the costimulatory molecules required to activate the adaptive immune response
- they have numerous characteristic "dendritic" processes branching from their prison cell membranes
- there are several specialised dendritic jail cell types, including Langerhans cells in the peel
Dendritic cells
LYMPHOCYTES
Modest, specialised white blood cells with large nuclei and no granules
- usually make upwards 20-45% of all white blood cells (1.3-3.5 x ten9/Fifty on a full blood count)
- at that place are three chief subtypes of lymphocytes: B cells, T cellsand natural killer (NK) cells
- B cells and T cells make up the majority of the lymphocyte population. They are modest cells with large round nuclei, scanty blueish-ish cytoplasm and no granules, and are morphologically duplicate from ane some other. The but way to tell them apart is with specialist serology or staining for specific cell surface markers known every bit clusters of differentiation (CDs).
- NK cells are a larger, more archaic lymphocyte subtype which do comprise some granules.
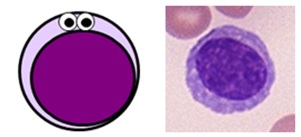
Lymphocyte
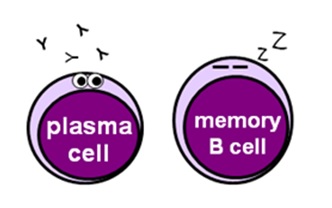
B CELLS
- B cells represent about 25% of the total lymphocyte population – this varies depending on the activeness of the allowed response and can be up to 50%
- important B cell surface markers includeCD19, CD20 and CD21, as well asMHC Ii
- they are essential for humoral immunity, besides known as the antibiotic-mediated immune response
- plasma cells are mature B cells that secrete antibodies, which recognise specific foreign antigens and bind to them or destroy them
- retentiveness B cells "think" the offending strange antigens to allow the immune organization to mount a quicker antibody response to whatsoever subsequent infections
T CELLS
- T cells represent about 70% of the total lymphocyte population – this varies depending on the activity of the allowed response and can be up to 90%
- all T cells express CD3 on their surfaces, along with T cell receptors (TCRs) which recognise specific antigens presented in an MHC I or MHC Ii molecule
- there are numerous different T cell subtypes with different roles, which each have their own identifiable surface markers – in that location are admittedly loads of these, and so I've merely discussed the nigh important ones below
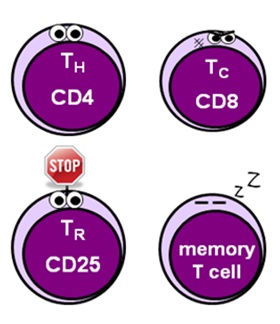
- helper T cells (CD4) facilitate the activation of the allowed response and stimulate division and differentiation of various effector cells
- cytotoxic T cells (CD8) – too known as killer or effector T cells – providecell-mediated immunity past targeting and killing infected cells
- regulatory T cells (CD25 + FOXP3) – also known as suppressor T cells – play a vital part in limiting the immune response to foreclose excessive damage to tissues and organs
- memory T cells (CD62 + CCR7) "remember" what has happened to allow the allowed system to mount a faster, more effective response should the offending organism be foolish enough to return
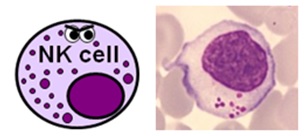
NATURAL KILLER CELLS
- NK cells represent about 5% of the total lymphocyte population – again, this varies depending on what'southward going on
- they are a larger, primitive lymphocyte subtype with granules in their cytoplasm – they are also known past haematologists as large granular lymphocytes (LGLs)
- they express CD16 and CD56, and a big proportion of them also express CD8
- NK cells actually class function of both the innate and adaptive immune systems and are able to destroy pathogens and infected cells without the need for prior activation by specific antigens.
- They are also peculiarly important in viral immunity and tumour rejection.
Natural killer cells
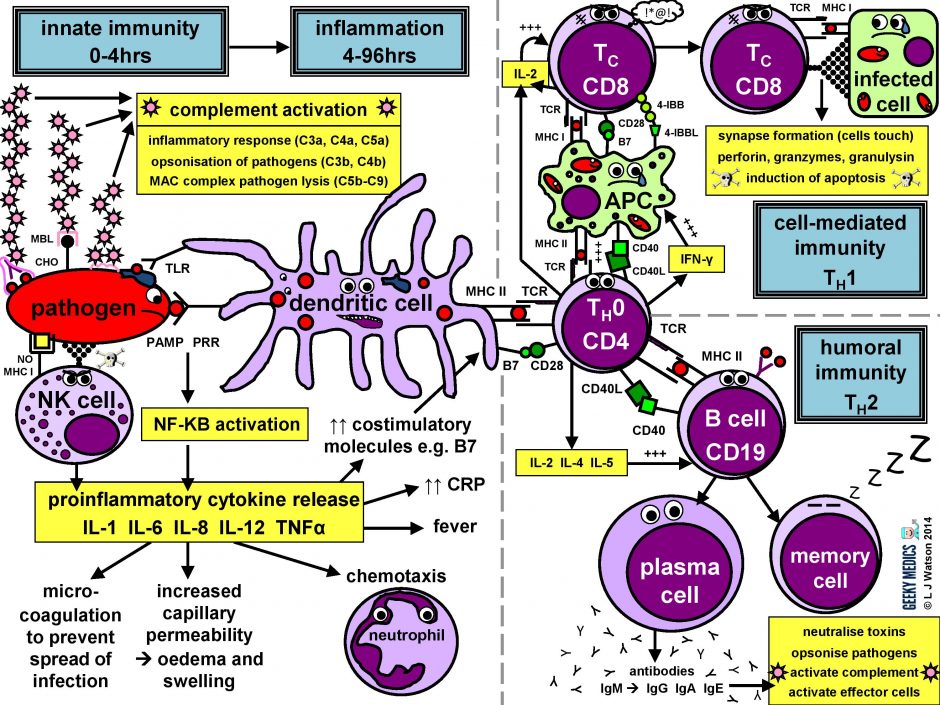
The immune response in a nutshell
The normal allowed response tin be cleaved down into four master components:
- pathogen recognition by cells of the innate allowed arrangement, with cytokine release, complement activation and phagocytosis of antigens
- the innate immune arrangement triggers an acute inflammatory response to contain the infection
- meanwhile, antigen presentation takes place with the activation of specific T helper cells
- CD4 helper T cells then co-ordinate a targeted antigen-specific immune response involving two adaptive cell systems:humoral immunity from B cells and antibodies, andcell-mediated amnesty from cytotoxic CD8 T cells
Let'south start at the very commencement. An evil pathogen has penetrated the torso's bulwark mechanisms and is plotting to start a nasty infection…
Part 1 – Innate immune arrangement
This is the first line of defence against any infection. Information technology is very fast – it is established within about iv hours – but is non-specific and has no retentivity, so it is not strong plenty to effectively tackle an infection on its own. It consists of a cellular response by the innate immune system, a chemical response past cytokines and complement, and the subsequent initiation of an astuteinflammatory response.
INNATE CELLULAR IMMUNE RESPONSE
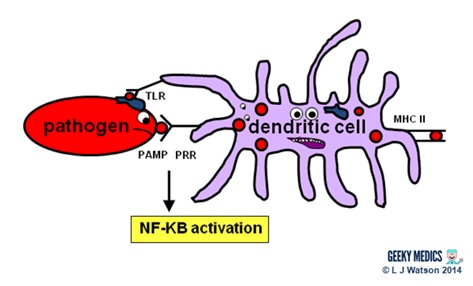
Phagocytes
Phagocytes are a very of import office of the innate allowed organization, as they act to fight the new infection and present antigens. Examples of "professional" phagocytes include dendritic cells, claret monocytes, tissue macrophages and, most importantly, neutrophils. Neutrophils are an admittedly key component of this initial process which just announced in response to infection or injury, and are therefore not found in good for you tissue.
- phagocytes place pathogens past recognising pathogen-associated molecular patterns (PAMPs) using pathogen recognition receptors (PRRs). Toll-similar receptors (TLRs) are an example of a PRR.
- one time they have identified dangerous organisms, they internalise them, kill them and assimilate them downwards into their component proteins
- phagocytes and so nowadays the digested poly peptide antigens to the cells of the adaptive allowed organization via major histocompatibility complexes (MHCs) on their surfaces. The MHC complex acts every bit a safety machinery. It prevents the immune system from existence activated likewise easily, as it ensures that T cells can only react to an antigen if it is presented within an MHC complex. This phenomenon is known as MHC restriction.
- when phagocyte PRRs are exposed to PAMPs, NFKB is activated. This is a transcription gene which results in the release of proinflammatory cytokines and the initiation of the inflammatory response.
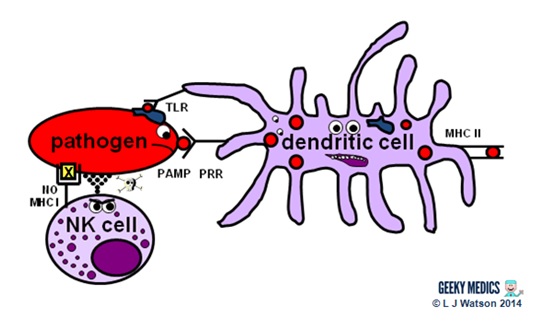
Natural killer cells (NK cells)
Natural killer cells, along with neutrophils and other phagocytes, also have an important role in the initial front-line defence against the infection.
- unlike T cells, they do not require activation by specific antigens, which means they are able to respond immediatelywhen exposed to a pathogen
- "cocky" cells are protected from the destructive action of NK cells by the inhibitory effects of MHC I, which is expressed on the surface of all nucleated trunk cells
- any cells without an identifiable MHC I are likely to exist "non-self" and fair game for firsthand anything – NK cells do this by releasing toxic granules to induce apoptosis
- normally, NK cells cannot set on healthy "cocky" cells. However, MHC I expression is oft suppressed if cells are infected with viruses, or have get malignant. NK cells tin, therefore, perform additional vital roles in viral immunity and tumour rejection.
INNATE CHEMICAL IMMUNE RESPONSE
Complement system
Complem ent is a pour of chemicals similar to the clotting cascade.
There are three separate pathways that activate the complement arrangement:
- classical pathway: activated by antibody-antigen complexes (a.m.a immune complexes) on pathogen surfaces
- mannose-binding lectin pathway: activated when mannose-binding lectin binds to the carbohydrate molecule mannose on pathogen surfaces
- alternative pathway: C3 reacts directly with pathogen surfaces
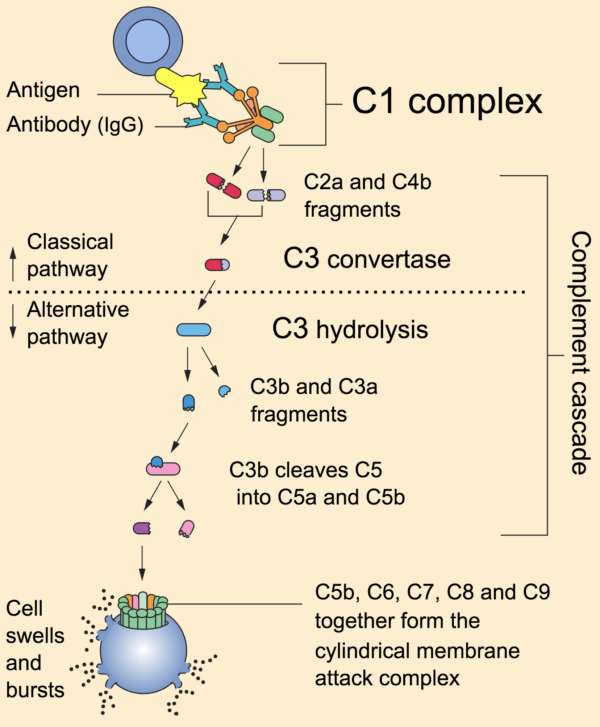
All iii of these pathways act to generate the enzyme C3 convertase. This cleaves C3 into two parts (C3a and C3b) and activates the rest of the cascade.
- C3a, along with C4a and C5a, is a mediator of inflammation which augments the inflammatory response. These molecules are also anaphylotoxins which trigger mast cell degranulation, histamine release and further inflammation.
- C3b binds to and coats pathogens, making them easier for phagocytes to identify and ingest. This process is chosen opsonisation. It also binds to immune complexes to facilitate their removal by the spleen and triggers the production of terminal components including C5b.
- C5b initiates the membrane attack pathway or "terminal lytic sequence". This triggers the formation of a membrane assail complex (MAC) made from C5b, C6, C7, C8 and C9. MACs are ring-shaped and essentially punch a hole in the pathogen cell membrane, resulting in osmotic lysis.
- complement mainly provides bacterial immunity. In viral infections, interferons play a similar function.
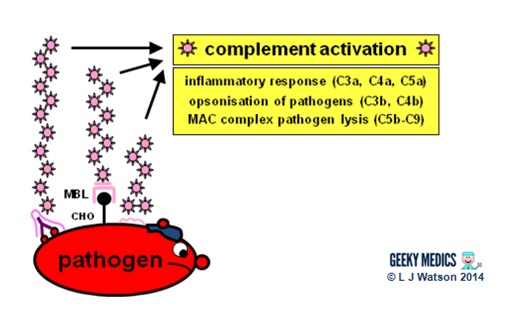
Proinflammatory cytokin es are the 2nd key component of the innate chemical allowed response. They are small messenger proteins released by immune cells in response to evidence of infection, which collaborate to mediate the astute inflammatory response (see Part 2). In that location are a huge number of different cytokine molecules, including whole families of interleukins, tumour necrosis gene s and chemokines.
Some of import examples include:
- IL-1 – causes fever and activates lymphocytes
- IL-six – causes fever, stimulates the liver to produce astute-stage proteins such as CRP, activates lymphocytes and promotes antibody production
- IL-8 (a.k.a CXCL8) – causes neutrophil chemotaxis
- IL-12 – activates NK cells and TH1 cells (of import for intracellular infections)
- TNF-alpha – increases vascular permeability to allow allowed cells to reach tissues
- IL-iv, IL-5 + IL-13 – promote IgE production and eosinophilic reactions in patients with allergies
- Interferon gamma (IFNγ) – essential in activating cell-mediated immunity in viral infections
- IL-10 – has an anti-inflammatory result
Part 2 – Inflammatory response
The acute inflammatory response is kick-started past innate allowed cells, proinflammatory cytokines and complement. It acts as a bridging mechanism to localise and contain the infection in the period from nearly four-96 hours after its onset, when the innate immune organization is running out of steam and the specific cellular immune response is withal trying to get going.
The main features of this process are:
- vasodilation and increased blood flow – this leads to erythema ("rubor") and warmth ("calor")
- increased vascular permeability – this allows an inflammatory cell infiltrate to extravasate and reach the site of infection, and also causes tissue oedema and swelling ("tumour")
- release of inflammatory mediators such as bradykinins and prostaglandins which increase pain sensitivity and crusade hyperalgesia in the infected area ("dolor")
- neutrophil chemotaxis – neutrophils migrate to the site of infection and begin their clean-up operation, phagocytosing pathogens and debris
- microvascular coagulation – this is induced past local tissue damage, and acts to confine the infection and prevent its spread
- systemic features such equally feverand raised inflammatory markers such every bit CRP and ferritin –this produces unpleasant "influenza-like" symptoms such as hot flushes, sweats, chills, rigors, headache, nausea, myalgia, arthralgia and fatigue.
- upregulation of costimulatory molecules such as MHC-2 and B7 to encourage activation of the adaptive immune system
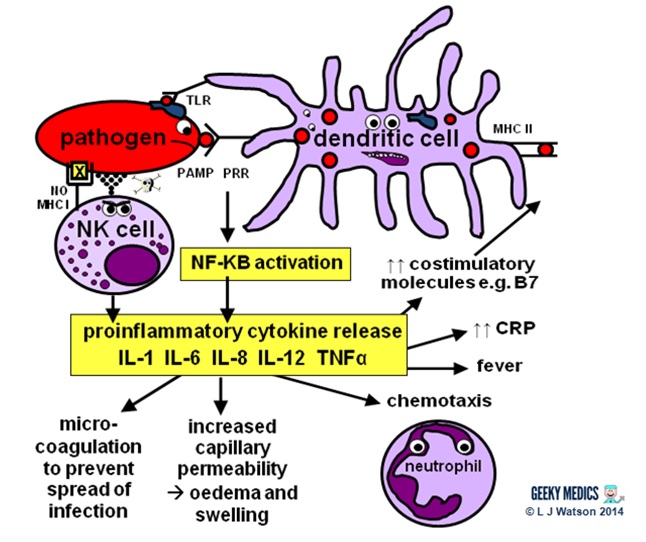
Part 3 – Antigen presentation
The innate immune arrangement and inflammatory response can only hold off an infection for so long – ultimately, a specific immune response needs to be activated. This is done via antigen presentation to the adaptive immune system.
- dendritic cells laden with digested antigens travel via the circulation to lymph nodes
- once they arrive at that place, they outset to present their antigens to naive T helper cells (TH0) withinMHC IIcomplexes on their prison cell surfaces
It is very important that the immune response is non activated inappropriately, as this could cause a lot of unnecessary damage. In that location are two primary protective mechanisms which foreclose this from happening by controlling the activation of the adaptive immune system:
- MHC brake ensures that but antigens presented within the context of MHC complexes are able to trigger the immune response
- in lodge to become fully activated past their specific antigen, naive T helper cells too require asecond signal from antigen-presenting cells. Dendritic cells are able to provide this in the form of B7 proteins (CD80 or CD86) which demark to CD28 receptors on T cell surfaces.
- expression of second point molecules is increased by the presence of an inflammatory response, increasing the likelihood of T helper cell activation
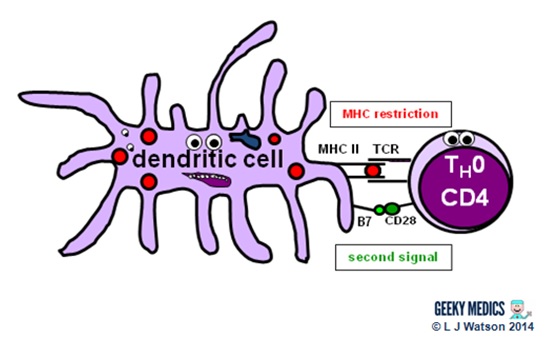
The combination of the correct antigen, anMHC 2 and a B7 2d signal gives the green low-cal for naive T helper cells to go going. The side by side pace is for them to differentiate into eitherTH1 cells, which promote cytotoxic T cells and jail cell-mediated immunity, or TH2 cells, which promote B cells and humoral immunity.
Part 4a – Humoral immunity
Humoral immunity is the term for a specific adaptive immune response activated past TH2 cells, which leads to the production of B cells and antibodies.
This allowed response is designed to fightextracellular infections, including near bacteria and fungi, protozoans such every bit Giardia, and parasitic worms such as Schistosoma.
ANTIBODIES
Antibody molecules are essentially secreted B prison cell receptors which provide an antigen-specific action. They are Y-shaped molecules with a complex structure comprising:
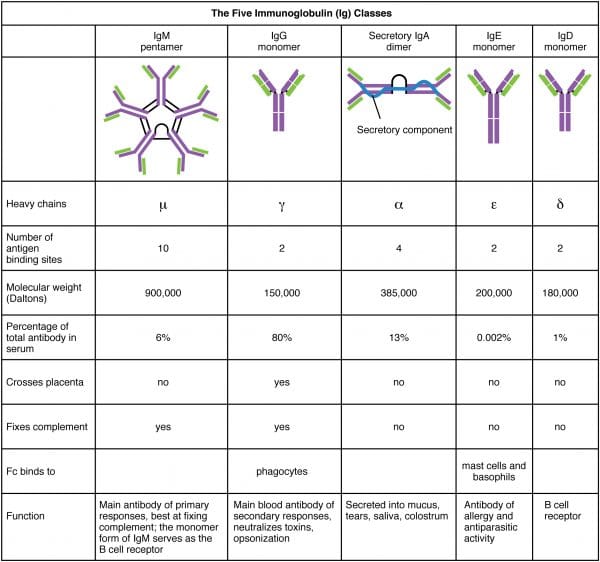
- two large heavy chains – their structure dictates whether the antibody is IgM, IgG, IgA, IgE or IgD
- two minor light chains – these can be either "kappa" or "lambda" types
- the heavy and light bondage are continued by disulphide bonds
- all four chains consist of constant and variable regions: the constant "C" regions are ever the same, but the variable "Five" regions are unique to each B cell and confer antigen specificity
- antigens bind to the ends of each "arm" of the Y structure
- the base of the antibody binds to complement and phagocytes
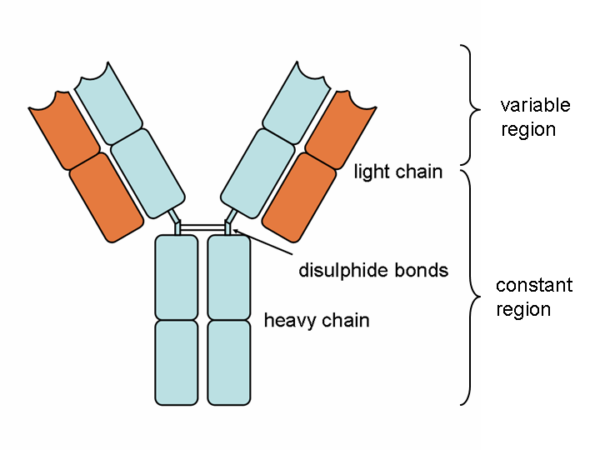
There are five antibody classes or "isotypes". These are dictated by the structure of the heavy chain abiding region.
- IgM – this has a pentameric structure. It is expressed on B prison cell surfaces and produced early in the immune response whilst IgG is being generated.
- IgG – this has a monomeric structure and provides the majority of antibody-based amnesty. It is institute mainly in circulating claret and tissues (it also crosses the placenta to provide passive immunity to the fetus).
- IgA – this forms a dimeric structure once it reaches its target tissues. Information technology is establish in mucosal areas such as the GI, respiratory and urinary tracts. Information technology is also secreted in saliva, tears and breast milk.
- IgE – this has a monomeric construction. It binds to allergens and mediates allergic reactions, equally well as providing amnesty confronting multicellular organisms such equally parasitic worms.
- IgD – this has a monomeric structure and is rather mysterious. It is found in very low levels in the serum, and appears to interact with basophils and mast cells.
At that place are a potentially infinite number of antigens the immune system might encounter, so it is vital that the immune response is able to adapt to this and reflect a similar degree of diversity. Humans demand to exist able to generate about 10 billion different antibodies, only information technology would be impossible to code every possible antibody into the human genome and still be able to fit our DNA into such tiny spaces within cells. As a result, a range of mechanisms have evolved to allow B cells to manipulate their own DNA and produce billions of different variable region structures:
- antibiotic variable region genes are coded in iii parts: Five (variable), D (diversity) and J (joining) segments. RAG proteins permit B cells to shuffle these gene segments around during their maturation and recombine them in millions of different means. This is known equally VDJ recombination.
- junctional variety is produced by the imprecise joining of VDJ segments during maturation, equally the variable overlap of genes results in the gain or loss of a few nucleotides
- "looping out" and rejoining of factor segments produces variations in genomic structure
- genetic diversity is too increased by the addition of random nucleotides calledNorthward regions
- when mature B cells are activated past their specific antigen, they get-go to produce IgM antibodies, and also undergo isotype course switching to produce different types of antibody adapted for various locations within the body
- B cell activation also promotes somatic hypermutation of variable region genes to produce always-so-slightly different versions of the same specific antibody. These are "tested" to observe the best match using clonal selection, and the ones with the highest possible affinity for the antigen are encouraged to proliferate in a process chosen affinity maturation.
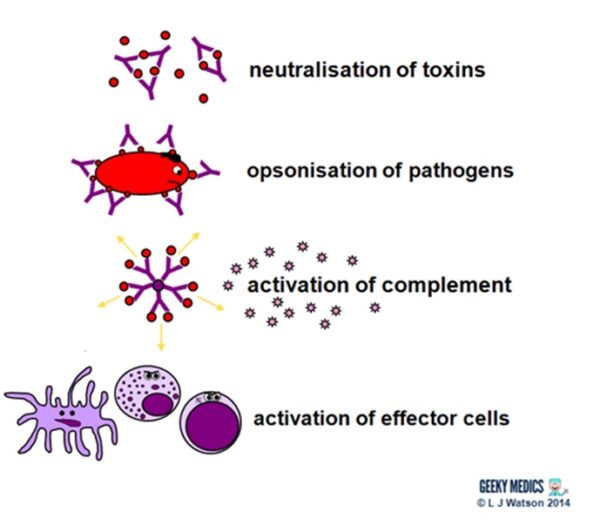
Antibodies fight extracellular infections in a number of ways:
- they neutralise toxins by directly binding to them
- they bind to antigens onpathogen surfaces. Thisagglutinates them to impair their mobility and too opsonises them to enhance phagocytosis.
- the bounden ofantibodies to antigens to class complexes activates the classical complement pathway
- they also directactivate effector cells such every bitdendritic cells,NK cells andcytotoxic T cells
THE HUMORAL IMMUNE RESPONSE
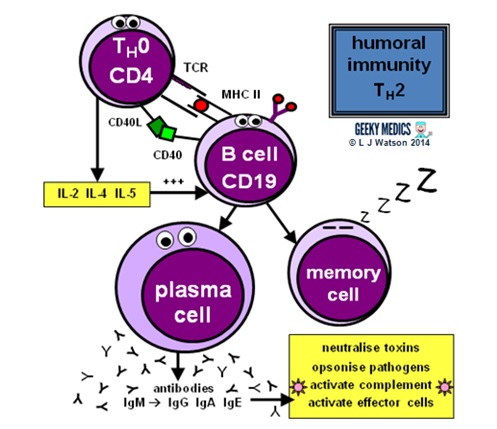
Humoral immunity and antibody production are dependent upon T helper cells activating B cells:
- once naive TH0 cells have been activated by their specific antigen, they differentiate into TH2 cells
- TH2 cells locate their respective B jail cell counterparts by identifying the correct antigen within an MHC II on the B cell'due south surface
- they then provide the B cell with a second signal, in this example, CD40 ligand which binds to CD40 on the B cell surface
- they also release cytokines such as IL-2, IL-four and IL-5 which promote B jail cell development
Activated B cells mature into plasma cells and outset to make antibodies:
- initially, plasma cells produce IgM antibodies, then isotype class switching produces dissimilar types to cover different areas of the body
- clonal expansion of antigen-specific plasma cells is followed bysomatic hypermutation,clonal selection andaffinity maturation to ensure the production of the all-time antibodies for the job, which are so released to tackle an infection
Once the infection has been cleared, some plasma cells volition remain asdormant "memory" B cells:
- only the well-nigh highly-antigen specific B cells produced by affinity maturation will be selected to become memory cells
- the presence of retention cells ways that immediate plasma cell proliferation and antibiotic production can occur at the time of the adjacent infection
- the number of surviving memory cells increases after each reinfection, so the more times you are exposed to a particular pathogen, the better your immune response to it becomes
Part 4b – Cell-mediated immunity
Cell-mediated immunity is the term for a specific adaptive immune response activated by TH1 cells, which leads to activation of antigen-presenting cells and acytotoxic T cell response.
This immune response is designed to fight intracellular infections, including viruses, some bacteria and fungi, andprotozoans such as PlasmodiumandToxoplasma.
T CELL RECEPTORS
T prison cell receptor diversity is just as important as antibiotic diversity, equally both systems demand to be able to embrace any possible infectious antigen. T cells generate this genetic diversity in essentially the same way equally B cells exercise during their evolution in the bone marrow, using VDJ recombination by RAG proteins, junctional diversity and the add-on of N regions, but they do non undergo grade switching or somatic hypermutation during their maturation.
All immature T cells undergo a rigorous "pedagogy" in the thymus gland earlier they are released into the bloodstream, but this process is especially important for cytotoxic T cells due to their subversive nature. They are "tested" with a variety of cocky cell antigens, and any cells which accept generated a receptor that reacts to these undergo negative selection and are destroyed. This essential mechanism prevents the immune system from reacting to the body and is known as immunological tolerance or cocky-tolerance. In order to graduate successfully from the thymus, T cells must also express CD3 and CD4 or CD8 (simply never both), and bind to self MHC complexes (simply not as well strongly). Merely virtually 1% of T cells generated in the bone marrow actually make information technology through this process alive!
ACTIVATION OF ANTIGEN PRESENTING CELLS
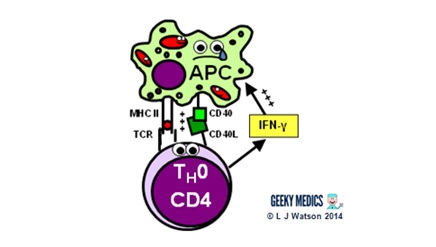
The start step of the prison cell-mediated allowed response is the activation of antigen-presenting cells:
- a TH1 cell encounters an unhappy infected antigen-presenting cell and recognises the MHC 2-restricted antigen on its surface
- it then "activates" the APC by providing a CD40 ligand second indicate and secreting interferon-gamma(IFNγ), a cytokine which is essential in stimulating the allowed response to intracellular infections
- once activated, APCs are able to increase their production of nitric oxide and superoxide radicals, which optimises their killing mechanisms and allows them to destroy ingested pathogens much more effectively
THE CYTOTOXIC T Prison cell RESPONSE
The side by side footstep is the activation of anantigen-specific cytotoxic T cell response:
- activated APCs nowadays their antigen to the specific cytotoxic T jail cell receptor within an MHC I, along with a diverseness of second signals, including B7 + CD28 and/or four-IBB + 4-IBBL
- this procedure is helped along by the secretion of IL-ii – a stiff T cell growth factor– by TH1 cells and the cytotoxic T cells themselves
In one case activated, the cytotoxic T cells are very smashing to become out and kickoff hunting and killing things. They identify infected cells by recognising the antigen displayed within MHC I on their surfaces. They and so destroy these cells using 1 of several mechanisms:
- they classically form an immunological synapse with their target cell – this simply ways the cell membranes touch – and release a substance called perforin to brand a hole in the prison cell wall. They then utilise this hole to release granzymes and granulysin into the cell, which induce apoptosis and Dna fragmentation.
- Fas ligand interactions between the cell surfaces can besides produce apoptosis of the infected cells via the aptly named death-inducing signalling complex (DISC)
- cytotoxic T cells tin can likewise release interferon-gamma(IFNγ), whichhas an interesting role in viral infections, as it is able to block intracellular viral replication without killing the cell itself. This effect is very useful, equally killing and lysing virally infected cells would simply allow all the baby viruses out and crusade the infection to spread itself even farther, which is clearly suboptimal.
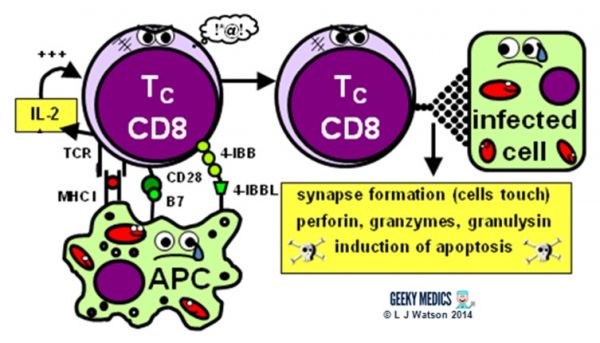
After the infection has been dealt with, the most antigen-specific cytotoxic T cells will remain behind as fallow retentivity T cells. The principles of T prison cell memory are essentially the same as B prison cell memory.
- during reinfection, just the outset signal (MHC + antigen) is required to activate the cytotoxic T cell response; no second signal is necessary
- this means that any antigen-presenting cell (not just dendritic cells) tin can activate cytotoxic T cells straight, reducing the need for TH1 cell help and resulting in a much swifter and more efficient prison cell-mediated allowed response.
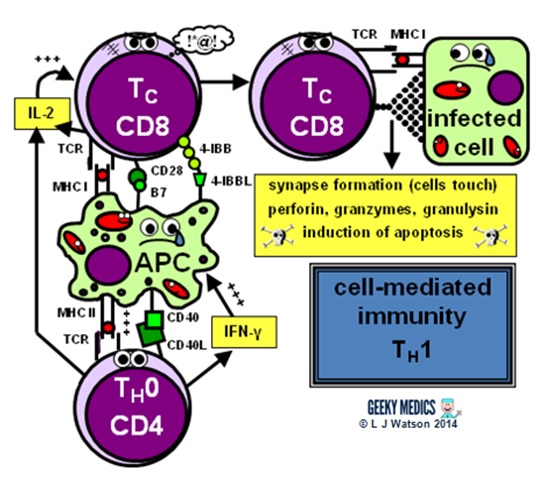
Summary of the immune response
We've now covered everything in the diagram in detail – hopefully it seems a lot less scary now!

Responses to dissimilar infections
Information technology is useful to be able to utilize your knowledge of the immune response to dissimilar types of infection, especially when it crops upward in exam questions. The most of import differentiation to brand is whether the infection is intracellular or extracellular, equally this generally dictates which branch of the adaptive immune response will exist activated:
- extracellular infections –>TH2 –>humoral allowed response withB cells and antibodies
- intracellular infections –>TH1 –> cell-mediated allowed response with activated APCs and cytotoxic T cells
Some types of pathogens can only exist every bit either extracellular or intracellular organisms, whilst other types can vary depending on the individual species. There are also unique variations in aspects of the immune response for some organisms.
BACTERIA
- bacterial infections trigger the classic allowed response as described in the master article above
- bacterial infections are usually extracellular
- however, some leaner exercise choose to exist equally intracellular organisms; examples of these includeNeisseria,Salmonella, Chlamydia and Mycobacteria
VIRUSES
- viral infections areintracellular and therefore handled by cell-mediated amnesty
- interferons are a family unit of cytokines which act as the equivalent of complement in viral immunity, and too have boosted unique functions. For example, cytotoxic T cells release interferon-gamma, which inhibits viral replication within infected cells without damaging the cells themselves.
- new infant viruses are released from cells as part of the spread of a viral infection, and viral antigens are as well expressed on the surfaces of infected cells. This means that some aspects of humoral immunity are also useful in viral infections. Antibodies are able to bind to viral antigens in order to neutralise and opsonise the infant viruses after they are released, limiting the spread of infection.
- natural killer cells besides play a vital function in viral amnesty, as they recognise and destroy infected cells which have either expressed antigen on their surfaces or lost their inhibitory MHC I
FUNGI
- the normal immune response is able to deal with fungal infections very swiftly and finer
- fungal infections are usuallyextracellular and therefore dealt with byhumoral immunity
- less normally, fungi opt to "suspension the mould" (deplorable) and become intracellular – examples of these include Histoplasma, Cryptococcus and Pneumocystis, all of which are well known to cause opportunistic infections in immunosuppressed patients defective thecell-mediated amnesty vital in tackling such organisms
- macrophages and other phagocytes are very important in fungal amnesty
PROTOZOANS
- our immune response to protozoa isn't that bang-up. This is probably considering many species have evolved hundreds ofclever protective mechanisms which turn the immune system to their advantage. Examples of protective mechanisms include resistance to phagocytosis and complement lysis, antigen variation, antigen shedding and fifty-fifty direct modifications of host immune mechanisms.
- examples of extracellular protozoa include Giardia, which infects the intestines and may have caused many of you lot the joys of traveller's diarrhoea; Entamoeba, which causes dysentery and nasty amoebic liver abscesses; and Trypanosoma, which causes sleeping sickness and featured in an first-class episode of Firm.
- other protozoa are intracellular organisms. Examples include Plasmodium, which occupies red blood cells and liver cells to cause malaria;Leishmania, which survives inside phagocytes subsequently being ingested and afflicted many soldiers during the conflicts in Republic of iraq and Afghanistan; andToxoplasma, which lives in many trunk tissues and can cause "crazy cat lady syndrome" amongst other things.
WORMS (a.thousand.a HELMINTHS)
- worms are very big compared to other infective organisms and are obviously e'er extracellular – common examples include Schistosoma, which spreads worms through the bloodstream to vital organs; Onchocercha which causes river blindness; and Taenia tapeworms which can cause malnutrition and cysticercosis
- TH2 cells and humoral immunity form the ground of the torso's immune response to parasitic worms
- eosinophils and IgE are also very important in killing helminths, as alongside promoting a powerful inflammatory response, they appear to bind to the opsonising antibodies on the worm'due south skin in club to later on dissolve information technology and impale information technology
The finish
I hope you found this guide helpful and fun. I certainly enjoyed creating the little allowed cells and telling their story (perhaps a scrap too much actually). It was a mammoth undertaking to write this thing, and while I have tried to exist as thorough and accurate as possible if any of you lot clever folks out there have noticed any mistakes/miscommunications/other cock-ups please do allow me know and then I can correct them for the benefit of everyone else. In return, you shall receive the advantage of your name immortalised below and a Geeky Medics gilded star…
- many thank you to Colin Hill for helpfully highlighting a mix-up in Part 3!
References
- Immune Organisation Anatomy. Past OpenStax College [CC BY three.0], via Wikimedia Eatables. Bachelor from: [LINK]
- Andy Gennery'south totally splendid immunology lectures given at Newcastle Medical School in 2009 and 2011 (I hope I did them justice)
- Potato K; "Janeway'south Immunobiology, 8th Edition", Garland Science.
- ParaSite – Parasites and Pestilence by Stanford University. Available from: [LINK]
- Patient UK. Full Blood Count. Bachelor from: [LINK]
Source: https://geekymedics.com/immune-response/

Post a Comment for "Describe the Effector Cells Involved in Humoral Immunity Review Sheet 35"